

We demonstrate successful construction and expression of three different co-expression systems, the proteosomal lid complex, the anaphase promoting complex/cyclosome (APC/C), and a series of constructs used to test the effect of chaperone co-expression on the solubility of the HOIP protein.

Individual expression constructs can therefore be combined into a single vector in a single reaction, with over 90% efficiency when combining up to 14 expression cassettes. In the same reaction, the cassettes are then ligated in the correct sequence in a final destination vector to generate multi-gene expression constructs containing 2–15 genes. This series of vectors can be cut by BsaI to excise cassettes with unique overhangs.
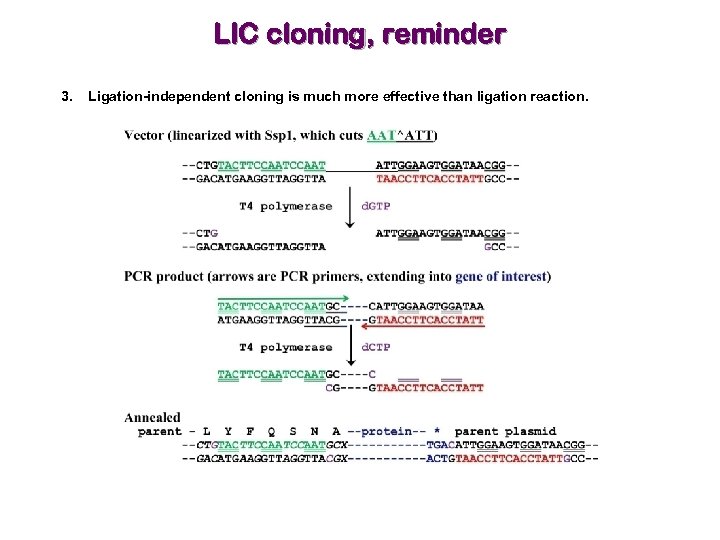
This method is based on the construction of a series of vectors containing a promoter-gene of interest-terminator cassette flanked by cleavage sites of the BsaI type IIS restriction enzyme. Here, we describe a rapid and simple Golden Gate-based system for the generation of multi-gene expression constructs compatible with baculovirus expression vector systems (BEVS) using either Tn7 transposition or KO1629-based homologous recombination, which we refer to as “GoldenBac”. These methods are multi-step, have lower efficiencies than single gene cloning, and may require laborious processes to verify that all genes of interest are present in the final product. Current methods are based either on serial rounds of combination of several vectors containing loxP sites via the Cre-lox technology, or on multiple rounds of gene combination via PCR or other methods. Recombinant protein production and purification of large protein complexes in eukaryotes requires efficient methods to generate multi-gene expression constructs, where each individual gene is under the control of its own promoter and terminator.


 0 kommentar(er)
0 kommentar(er)
